Treatment of citrin deficiency by in vivo gene editing
Citrin deficiency, resulting from mutations in the SLC25A13 gene, is a monogenic liver disease that impacts infants, children, and adults in diverse ways, leading to considerable morbidity and mortality. Although considered rare globally, its prevalence is high in East Asia, with a carrier rate of 1:65 in the Japanese population. Currently, no cure exists for this condition, and treatment is primarily based on a low-carbohydrate, lactose-free, and MCT-enriched diet.
In the past few years, our laboratories have played a pioneering role in the development of in vivo base editing approaches in the liver. Using lipid nanoparticle (LNP)-mediated delivery of RNA encoding for base editors, we were able to correct a mouse model for phenylketonuria (PKU). In addition, we employed the technology to install a mutation in PCSK9 in the liver of mice and non-human primates (NHPs), leading to a significant reduction of LDL-cholesterol levels in treated animals. Importantly, Verve Therapeutics, a US base biotech company, is currently conducting a clinical trial in hypercholesterolemia patients using this approach. More recently, we developed in vivo prime editing approaches in the liver. Prime editing is a novel "search-and-replace" genome editing technology, which similar to base editing works without DNA double strand break formation but is even more versatile, allowing the correction of all small-sized genetic changes. Although in vivo prime editing was less efficient than base editing, recent optimizations of the prime editor design led to a therapeutic correction of the PKU mouse model using LNP-mediated RNA delivery.
We will combine our expertise in urea cycle disorders and genome editing to develop a treatment for citrin deficiency as a model for genetic liver diseases. Towards this aim, we have performed a high-throughput screen in vitro in cell lines and identified two of the most frequently occurring pathogenic citrin deficiency mutations could be efficiently corrected via base- and prime editing. Thus, these two mutations are excellent candidates for developing base- and prime editing therapies. Our approach will be based on LNP-mediated RNA delivery, as it is ideally suited for translational genome editing in the liver: 1) LNPs and RNA can be produced at large scale, as demonstrated during the COVID pandemic, 2) the short half-life of mRNA ensures that the genome editor is only present for a limited amount of time, 3) own previous studies demonstrated high base editing rates in NHPs using this delivery system, and 4) clinical trials with classical CRISPR-Cas9 nucleases have shown that LNP-mediated RNA delivery into the liver is safe (Intellia Therapeutics). We propose the below described pre-clinical experiments, which we expect can be conducted within the next 3-4 years and which will lead to the start of a clinical trial
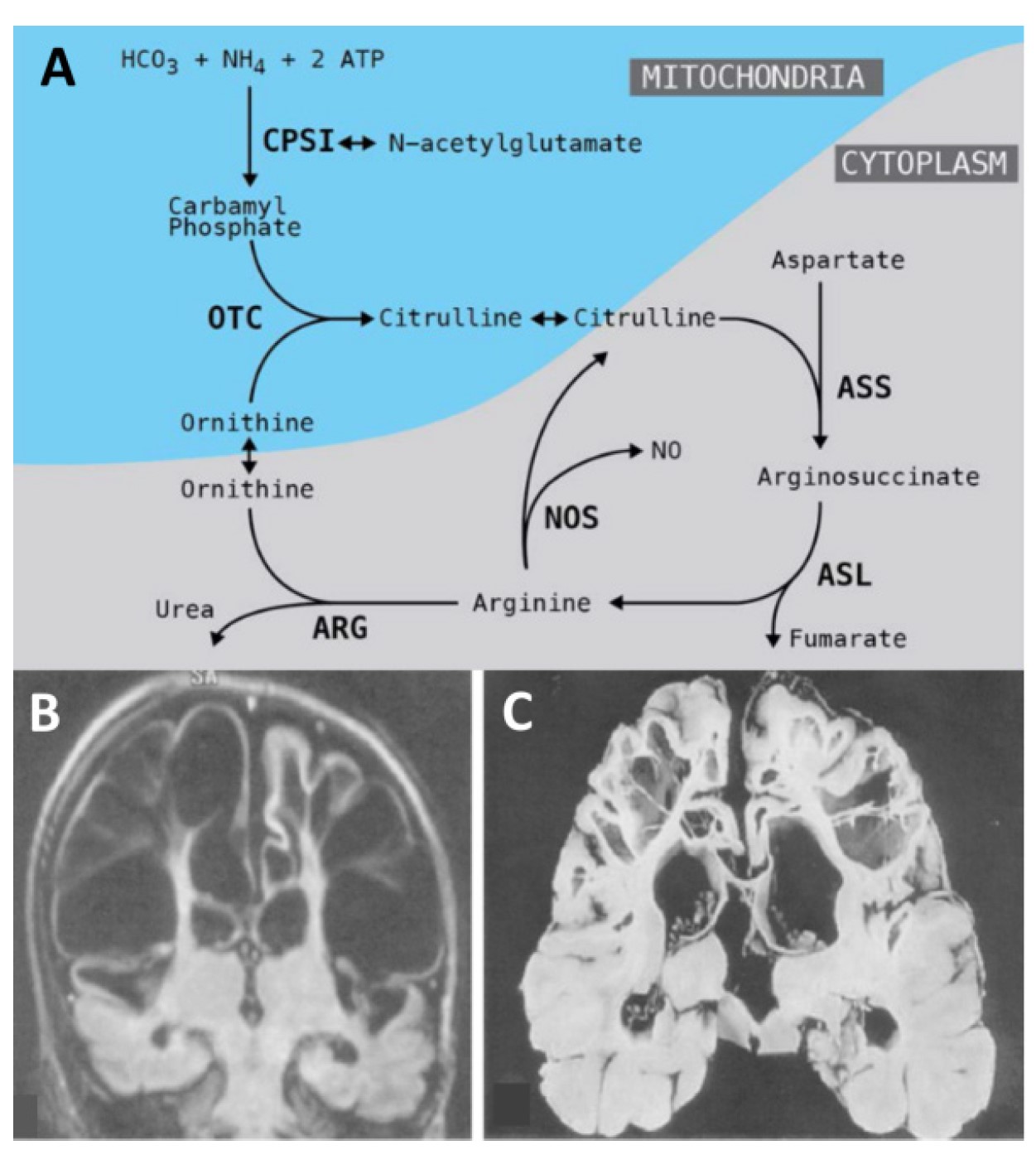
Figure 1. Urea cycle disorders A) The OTC enzyme is active in the mitochondria of liver cells. OTC deficiency causes malfunctioning of the urea cycle and ammonia accumulation in the blood (hyperammonaemia). B-C) Brain damage in OTC deficiency. Magnetic resonance B) and section of the brain at autopsy C) show multiple cysts in a patient with hyperammonaemia.