Research project on Vanishing White Matter Disease
Therapeutic use of CRISPR/Cas9 base editing for the treatment of Vanishing White Matter Disease
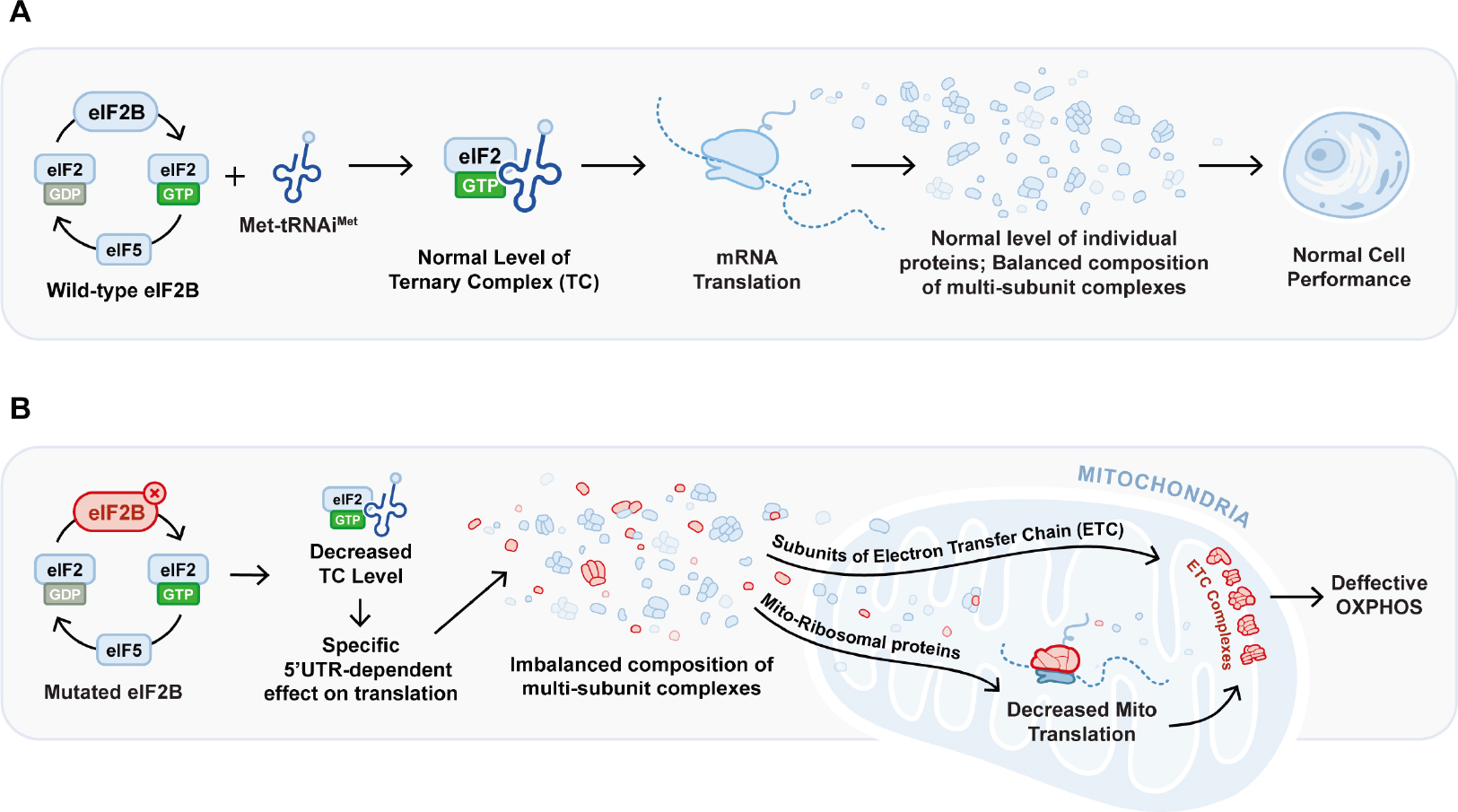
Leukodystrophies, such as Vanishing White Matter disease, are rare terminal brain disorders caused by the progressive loss of myelin in the central and peripheral nervous system. Since specific point mutations in the genes encoding the five subunits of the eukaryotic translation initiation factor 2B (Eif2B1-5) have been associated with onset and progression of myelin degeneration in VWMD, in vivo CRISPR/Cas9-base editing constitutes a promising treatment approach to revert these disease-causing mutations. Nevertheless, in vivo genome editing in patients still faces several challenges, including immunogenicity of delivery vehicles and Cas proteins and generation of off-target mutations that could induce malignant transformations.
The proposed project aims to tackle several of the aforementioned challenges in order to establish base editing for the treatment of VWMD. Using a series of read-outs ranging from genomics, transcriptomics, proteomics to behavioural analyses, we will evaluate the therapeutic efficacy and long-term effects of a CRISPR/Cas9-base editing treatment in vivo in a VWMD mouse model. As there are currently no curative therapies for VWMD and various other genetic brain disorders, such as Rett Syndrome, Huntington’s disease and Niemann-Pick disease, the here-developed approach of base editing and targeted viral delivery holds immense potential for future therapeutic applications, also beyond leukodystrophies.