Gene Therapy for Glanzmann Thrombasthenia
Glanzmann Thrombasthenia (GT) is an inherited platelet function disorder caused by mutations in the ITGA2B or ITGB3 genes, encoding the integrin subunits αIIb (CD41) and β3 (CD61), respectively. These mutations lead to either a non-functional protein, reduced or absent expression of the integrin αIIbβ3 on megakaryocytes (MKs) and platelets, resulting in impaired platelet aggregation, thrombus formation, and clot retraction. Patients experience recurrent bleeding episodes, which can become life-threatening. Aside from symptomatic treatments, hematopoietic stem cell transplantation (HSCT) remains the only curative option available to date.
In our project, we aim to develop HSC-directed gene therapy as an alternative treatment for GT. In our approach, genetic modification will be done in the patient’s autologous HSC, and after their transplantation back into the patient, integrin αIIbβ3 expression in patients’ MKs and platelets will be restored. We will compare gene addition strategies by integrating the αIIb coding sequence into the endogenous ITGA2B gene locus by CRISPR/Cas9-mediated gene editing or by transduction with lentiviral vectors that express the αIIb coding sequence from MK-specific promoters. We will evaluate efficacy and safety of our gene therapy in vitro in suitable cell models of GT (cells line, HSC-derived MK, iPSC) and in vivo in a mouse model of Itga2b-deficient GT.
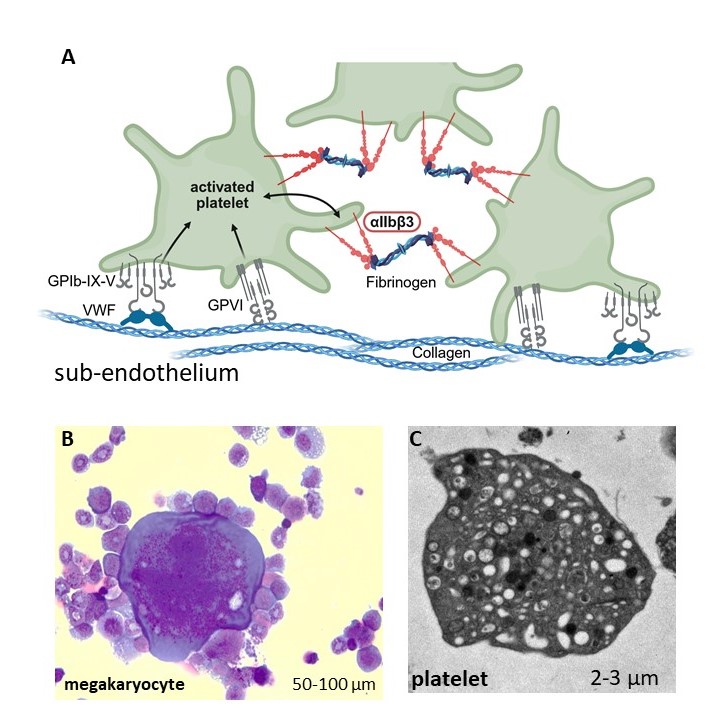
(A) Platelets attach to the subendothelial matrix on sites of vessel injuries. By binding of matrix components to platelet receptors, platelets get activated and initiate platelet aggregation via the aIIbb3 ntegrin, forming a blood clot. In Glanzmann Thrombasthenia, loss of aIIbb3 function impairs clot formation.
(B) Megakaryocyte from the bone marrow, May-Grünwald/Giemsa staining
(C) Electron microscopy of a mouse platelet; the anuclear cell is filled with alpha and dense granules that secrete bioactive substances after platelet activation